Alchemic Group
Seven Planetary Metals
Seven metals are associated with the seven classical planets, and seven deities, all figuring heavily in alchemical symbolism. Although the metals occasionally have a glyph of their own, the planet’s symbol is used most often, and the symbolic and mythological septenary is consistent with Western astrology. The planetary symbolism is limited to the seven wandering stars visible to the naked eye, and the extra-Saturnarian planets such as Neptune are not used.
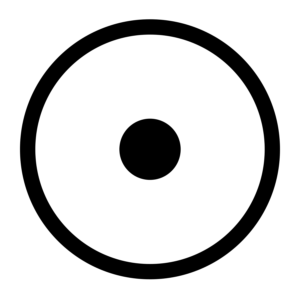
Gold is the most malleable and ductile of all metals; a single gram can be beaten into a sheet of 1 square meter, or an ounce into 300 square feet. Gold leaf can be beaten thin enough to become translucent. The transmitted light appears greenish blue, because gold strongly reflects yellow and red. Such semi-transparent sheets also strongly reflect infrared light, making them useful as infrared (radiant heat) shields in visors of heat-resistant suits, and in sun-visors for spacesuits.

Silver is a very ductile, malleable (slightly harder than gold), monovalent coinage metal, with a brilliant white metallic luster that can take a high degree of polish. It has the highest electrical conductivity of all metals, even higher than copper, but its greater cost has prevented it from being widely used in place of copper for electrical purposes. Despite this, 13,540 tons were used in the electromagnets used for enriching uranium during World War II (mainly because of the wartime shortage of copper). An exception to this is in radio-frequency engineering, particularly at VHF and higher frequencies, where silver plating to improve electrical conductivity of parts, including wires, is widely employed. Another notable exception is in high-end audio cables, where manufacturers claim that scaling copper conductors by 6% achieves slightly better results.
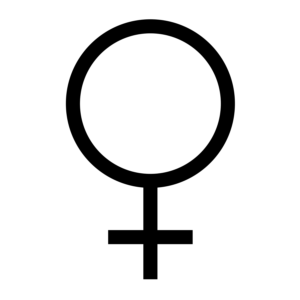
Copper forms a rich variety of compounds with oxidation states +1 and +2, which are often called cuprous and cupric, respectively. It does not react with water, but it slowly reacts with atmospheric oxygen forming a layer of brown-black copper oxide. In contrast to the oxidation of iron by wet air, this oxide layer stops the further, bulk corrosion. A green layer of verdigris (copper carbonate) can often be seen on old copper constructions, such as the Statue of Liberty, the largest copper statue in the world build using repoussé and chasing. Hydrogen sulfides and sulfides react with copper to form various copper sulfides on the surface. In the latter case, the copper corrodes, as is seen when copper is exposed to air containing sulfur compounds. Oxygen-containing ammonia solutions give water-soluble complexes with copper, as do oxygen and hydrochloric acid to form copper chlorides and acidified hydrogen peroxide to form copper(II) salts. Copper(II) chloride and copper comproportionate to form copper(I) chloride.
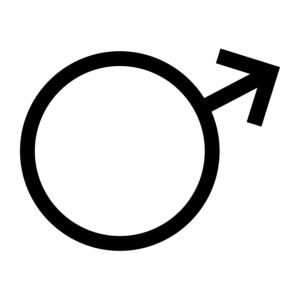
Iron is the sixth most abundant element in the Universe, formed as the final step of nucleosynthesis, by silicon fusing in massive stars. Metallic iron is rarely found on the surface of the earth because it tends to oxidize, but its oxides are pervasive and represent the primary ores. While it makes up about 5% of the Earth's crust, both the Earth's inner and outer core are believed to consist largely of an iron-nickel alloy constituting 35% of the mass of the Earth as a whole. Iron is consequently the most abundant element on Earth, but only the fourth most abundant element in the Earth's crust. Most of the iron in the crust is found combined with oxygen as iron oxide minerals such as hematite and magnetite. Large deposits of iron are found in banded iron formations. These geological formations are a type of rock consisting of repeated thin layers of iron oxides, either magnetite (Fe3O4) or hematite (Fe2O3), alternating with bands of iron-poor shale and chert. The banded iron formations are common in the time between 3,700 million years ago and 1,800 million years ago. About 1 in 20 meteorites consist of the unique iron-nickel minerals taenite (35–80% iron) and kamacite (90–95% iron). Although rare, iron meteorites are the main form of natural metallic iron on the Earth's surface. It was proven by Mössbauer spectroscopy that the red color of the surface of Mars is derived from an iron oxide-rich regolith.

Tin is generated via the long S-process in low-medium mass stars (with masses of 0.6 to 10 of that of Sun). It arises via beta decay of heavy isotopes of indium. Tin is the 49th most abundant element in the Earth's crust, representing 2 ppm compared with 75 ppm for zinc, 50 ppm for copper, and 14 ppm for lead. Tin does not occur as the native element but must be extracted from various ores. Cassiterite (SnO2) is the only commercially important source of tin, although small quantities of tin are recovered from complex sulfides such as stannite, cylindrite, franckeite, canfieldite, and teallite. Minerals with tin are almost always associated with granite rock, usually at a level of 1% tin oxide content. Because of the higher specific gravity of tin dioxide, about 80% of mined tin is from secondary deposits found downstream from the primary lodes. Tin is often recovered from granules washed downstream in the past and deposited in valleys or under sea. The most economical ways of mining tin are through dredging, hydraulic methods or open cast mining. Most of the world's tin is produced from placer deposits, which may contain as little as 0.015% tin. It was estimated in January 2008 that there were 6.1 million tons of economically recoverable primary reserves, from a known base reserve of 11 million tons.
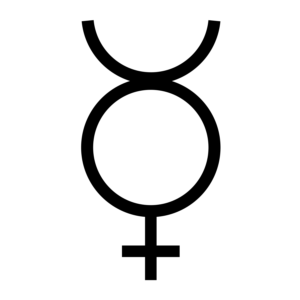
Mercury is an extremely rare element in the Earth's crust, having an average crustal abundance by mass of only 0.08 parts per million (ppm). However, because it does not blend geochemically with those elements that constitute the majority of the crustal mass, mercury ores can be extraordinarily concentrated considering the element's abundance in ordinary rock. The richest mercury ores contain up to 2.5% mercury by mass, and even the leanest concentrated deposits are at least 0.1% mercury (12,000 times average crustal abundance). It is found either as a native metal (rare) or in cinnabar, corderoite, livingstonite and other minerals, with cinnabar (HgS) being the most common ore. Mercury ores usually occur in very young orogenic belts where rock of high density are forced to the crust of the Earth, often in hot springs or other volcanic regions.
Lead is bright and silvery when freshly cut but the surface rapidly tarnishes in air to produce the commonly observed dull luster normally associated with lead. It is a dense, ductile, very soft, highly malleable, bluish-white metal that has poor electrical conductivity when compared to most other metals. This metal is highly resistant to corrosion, and because of this property, it is used to contain corrosive liquids (for example, sulfuric acid). Because lead is very malleable and resistant to corrosion it is extensively used in building construction – for example in the external coverings of roofing joints. Metallic lead can be toughened by addition of small amounts of antimony, or a small number of other metals such as calcium. All isotopes of lead, except for lead-204, can be found in the end products of the radioactive decay of the even heavier elements, uranium and thorium. Powdered lead burns with a bluish-white flame. As with many metals, finely divided powdered lead exhibits pyrophoricity. Toxic fumes are released when lead is burned.
